Temperature on Coffee Freshness: Insights from Arrhenius
Freshly roasted coffee delivers its full aroma only under optimal conditions. The Arrhenius Equation shows how chemical reactions, like oxidation, speed up with rising temperatures while reducing freshness. Did you know that coffee stored in the freezer at –20 °C ages about 12 times slower than at room temperature? Understanding the effects of temperature and proper storage can help you enjoy your favorite coffee longer, with its full flavor intact.
8/20/20252 min read


Understanding Coffee Freshness
The freshness of coffee is a critical factor that influences its flavor, aroma, and overall quality. As coffee enthusiasts, we often seek to enjoy the rich profiles that freshly roasted beans provide. However, the intricate relationship between temperature and coffee freshness is an essential component of this endeavor. One of the scientific frameworks that help us understand this relationship is the Arrhenius Equation.
The Arrhenius Equation Explained
The Arrhenius Equation, formulated by Svante Arrhenius in 1889, mathematically describes how the rate of a chemical reaction changes with temperature:
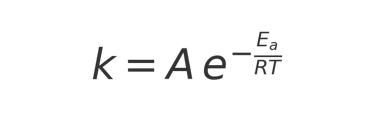
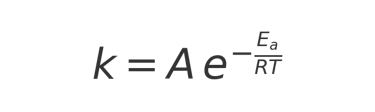
Where:
k = reaction rate constant
A = frequency factor (how often reacting molecules collide in the right orientation)
Ea = activation energy (minimum energy required for the reaction to occur)
R = universal gas constant (8.314 J·mol⁻¹·K⁻¹)
T = absolute temperature in Kelvin
In simpler terms, the equation shows that reaction rates increase exponentially with temperature. For coffee, this means that chemical reactions responsible for staling—such as oxidation and volatile aroma compound degradation—accelerate as storage temperatures rise.
Temperature Variations and Their Effects
Freshly roasted coffee beans are particularly vulnerable in warmer environments. Storing beans at room temperature in a hot kitchen can lead to a rapid loss of flavor and aroma, significantly affecting the brewing experience. Conversely, lowering the storage temperature slows down these unwanted reactions.
A general rule derived from the Arrhenius principle is that a 10 °C increase in temperature roughly doubles the rate of chemical reactions. This means coffee stored at 30 °C may age about twice as fast as coffee stored at 20 °C.
Freezer Storage and Coffee Aging
Several scientific studies have applied the Arrhenius framework to coffee aging. They consistently show that storing roasted coffee at around –20 °C slows aging by approximately a factor of 12 compared to room temperature.
In practical terms:
1 week at room temperature ≈ 3 months at –20 °C
This dramatic slowdown is why freezer storage is considered one of the most effective ways to preserve coffee freshness. Freezing reduces oxidation and volatile loss, helping the beans retain their aromatic complexity and flavor clarity for much longer.
Literature:
Yeretzian, C., Smrke, S., & Specialty Coffee Association. (Eds.). (2018). The Coffee Freshness Handbook (1st ed.). Specialty Coffee Association. Retrieved from https://sca.coffee/store/the-coffee-freshness-handbook
Arrhenius, S. (1889). Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren. Zeitschrift für Physikalische Chemie, 4(1), 226–248. https://www.degruyterbrill.com/document/doi/10.1515/zpch-1889-0416/html
MICROLOT
Microlot is microroastery based in Basel, dedicated to sourcing and roasting exceptional specialty coffee beans with transparency and care. Focused on light roasts, we work with suistanable and fair-trade-driven producers supporting the next generation of coffee growers. Every cup tells a story of quality to highlight the unique flavors of every origin.
© 2024. MICROLOT. All rights reserved.
